

Drug Facts |
Health & Safety |
Usage Instructions |
About this Product |
Company, Brand & Sustainability |
|
Adults and Children 12 Years of Age and Over
|
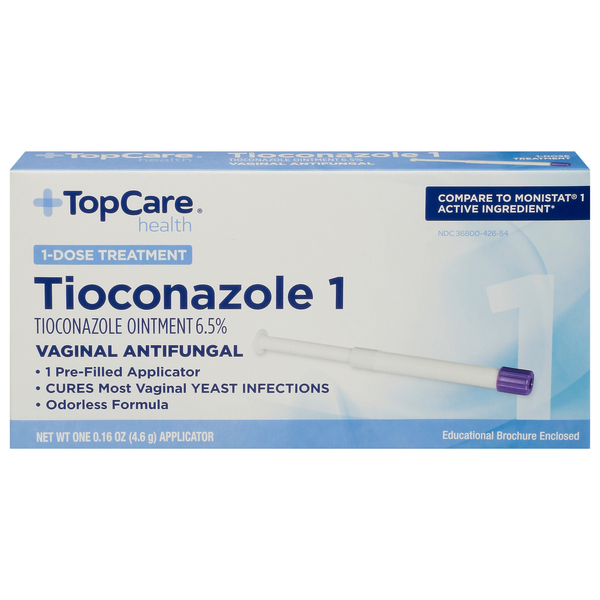
Open the printed packet just before use and remove purple cap. Insert entire contents of applicator into the vagina at bedtime. Throw applicator away after use.
|
|
Adults and Children 12 Years of Age and Over
|
Open the printed packet just before use and remove purple cap. Insert entire contents of applicator into the vagina at bedtime. Throw applicator away after use.
|
Product Allergens
Expiration Date
This product has an expiration date. Refer to expiration date stamped on packaging.
For vaginal use only.
Do not use if you have never had a vaginal yeast infection diagnosed by a doctor.
If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Tamper-evident unit do not use if sealed printed packet is torn, open or incompletely sealed.
About this Product
Certifications
SmartLabel® includes certifications from independent organizations that have meaningful and consistent standards for product composition, environmental protection and/or social justice.
Expiration Date
This product has an expiration date. Refer to expiration date stamped on packaging.
Product Allergens
ACTIVE INGREDIENTS
INACTIVE INGREDIENTS
Adults and Children 12 Years of Age and Over
Adults and Children 12 Years of Age and Over